Lab Reaction Rate :
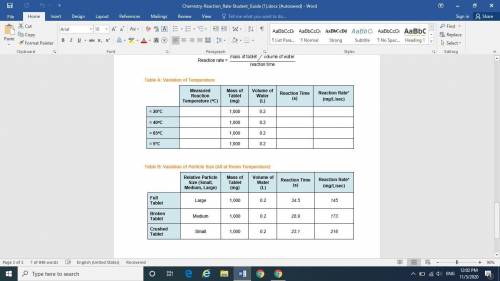
Variation of Temperature
Step 2: Measure the Reaction Rate at ≈ 20°C (Roo...

Chemistry, 05.11.2020 20:30 CloutLEVEL
Lab Reaction Rate :
Variation of Temperature
Step 2: Measure the Reaction Rate at ≈ 20°C (Room Temperature)
Step 3: Measure the Reaction Rate at ≈ 40°C
Step 4: Repeat Step 2, heating the water to Measure the Reaction Rate at ≈ 65°C
Step 5: Measure the Reaction Rate at ≈ 5°C
Variation of Particle Size
Step 6: Measure the Reaction Rate for a Full Tablet
Step 7: Measure the Reaction Rate for a Partially Broken Tablet
Step 8: Measure the Reaction Rate for a Crushed Tablet


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Questions in other subjects:


Chemistry, 03.10.2021 05:40



Mathematics, 03.10.2021 05:40








