
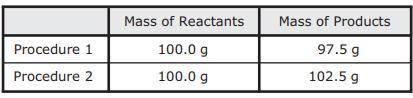
As part of an investigation, students combined substances in a beaker to observe chemical reactions. They performed two procedures. They measured the mass of each substance before and after each reaction. The table shows their observations.
Assuming the students did not make any careless errors, what likely explains these changes in mass?
A. Procedure 1: All the reactants were liquids that evaporated.
Procedure 2: A gas was formed as one product, and it escaped into the air.
B. Procedure 1: One of the reactants was converted to thermal energy.
Procedure 2: All the products were liquids.
C. Procedure 1: The reactants were liquids with different densities.
Procedure 2: The reactants were combined into only one product.
D. Procedure 1: One of the products was a gas that escaped into the air.
Procedure 2: A gas from the air reacted with one of the other reactants.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:20, montanolumpuy
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d. lytic
Answers: 1

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 17:40, Snowball080717
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
As part of an investigation, students combined substances in a beaker to observe chemical reactions....
Questions in other subjects:

Mathematics, 15.01.2021 02:10

English, 15.01.2021 02:10

Mathematics, 15.01.2021 02:10



Chemistry, 15.01.2021 02:10

Mathematics, 15.01.2021 02:10

Spanish, 15.01.2021 02:10


Physics, 15.01.2021 02:10



