
Chemistry, 04.11.2020 20:10 yeetmaster7688
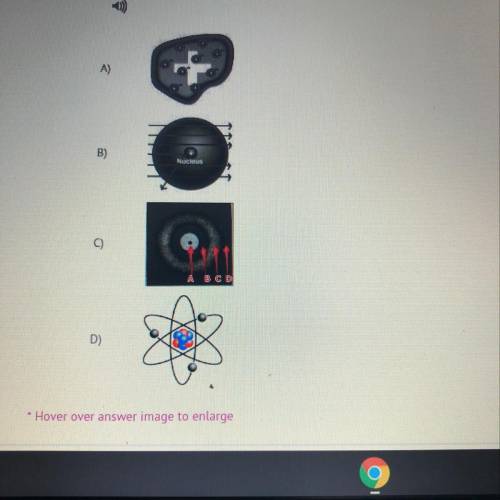
Thompson, Rutherford, Bohr, Planck, and Einstein all contributed models to help describe the atom and atomic
properties. Each model is an expression of the advancing research which improved our understanding the atom, its
subatomic properties, and properties by building upon previous research. A critical discovery involved explaining the Rydberg equation for the emission spectrum of hydrogen. Distinguish which one of these models was the first to relate the wavelength and quantized energy emissions.
Rydberg equation for the emission spectrum of hydrogen. Distinguish which one of these models was the first to
relate the wavelength and quantized energy emissions.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, angelrenee2000
Ineed someone to see if my answers are correct! if any are wrong let me know what the correct answers would be and how to get that answer! 1. how many moles of sodium chloride are in 28 grams od nacl? a. 265 mole naclb. 856 mole naclc. 479 mole of nacld. 1.2 mole nacl < my choice2. 734 grams of lithium sulfate (li2so4) are dissolved to make 2500 ml of solution what is rhe molaratiy? a. 2.67 mb. 4.56 mc. 3.89 m < my choiced. 1.78 m3. how many grams of cacl2 would be dissolved in 3.0 l of a 0.50 m solution of cacl2? a. 250 g cacl2 b. 166.5 g cacl2c. 113.65 g cacl2d. 98 g cacl2 < my choice4. suppose you had 58.44 g of nacl and you dissolved it in exactly 2.00 liters. the molarity if the solution would be 0.5 mtrue < my choicefalse 5. i would need 22g of naoh to make a 3.0 m solution using 250 ml of solvent. true < my choicefalse6. identify the solute: you have a .0195 m solution made from using 6.5 g of solute and 3 l of solvent. identify the solute by solving for molar weight. a. the solute is nacl because the molar weight is 58.43 g/mol < my choiceb. the solute is h2so4 because the molar weight is 98.06 g/molc. the solute is cacl2 because the molar weight is 111.11 g/mol
Answers: 1

Chemistry, 22.06.2019 11:30, ansuaprajita1506
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
Thompson, Rutherford, Bohr, Planck, and Einstein all contributed models to help describe the atom an...
Questions in other subjects:

Mathematics, 02.03.2021 05:30





History, 02.03.2021 05:30

Mathematics, 02.03.2021 05:30

History, 02.03.2021 05:30

Mathematics, 02.03.2021 05:30

Mathematics, 02.03.2021 05:30



