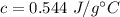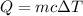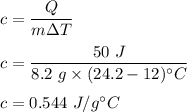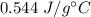Chemistry, 04.11.2020 18:40 abdullaketbi71
It takes 50.0 J to raise the temperature of an 8.20 g piece of unknown metal from 13.0∘C to 24.2 ∘C. What is the specific heat for the metal

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, ezrasedore
Identifying limitations of kinetic-molecular theorya chemist is studying the properties of a gas under various conditions. he observes that when the gas is at room temperature and low pressure, it behaves as an ideal gas. when the gas is cooled to 10 kelvin and is placed under high pressure, however, it deviates significantly from an ideal .
Answers: 1

Chemistry, 22.06.2019 04:00, speris1443
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 05:30, tifftiff22
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2
You know the right answer?
It takes 50.0 J to raise the temperature of an 8.20 g piece of unknown metal from 13.0∘C to 24.2 ∘C....
Questions in other subjects:



Social Studies, 16.11.2019 16:31

Mathematics, 16.11.2019 16:31


Geography, 16.11.2019 16:31


History, 16.11.2019 16:31

Biology, 16.11.2019 16:31