
Chemistry, 04.11.2020 08:10 smartowl101
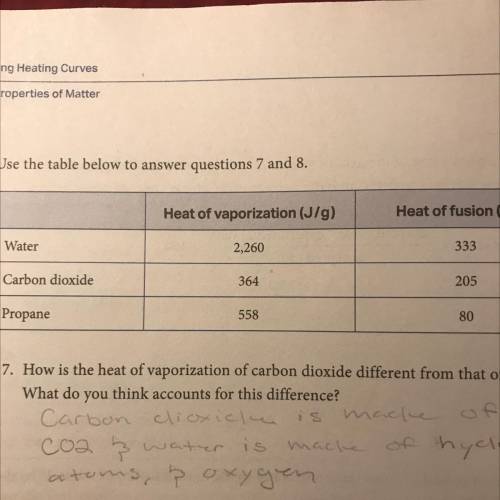
For water, propane, and carbon dioxide, compare the heat of fusion to the heat of
vaporization. What patterns do you see? Do you think these patterns hold true for
other substances as well? Why is vaporization greater than fusion in every case?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 23.06.2019 01:30, koggebless
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
For water, propane, and carbon dioxide, compare the heat of fusion to the heat of
vaporization. Wha...
Questions in other subjects:

Geography, 09.11.2019 04:31

History, 09.11.2019 04:31


Mathematics, 09.11.2019 04:31


Mathematics, 09.11.2019 04:31






