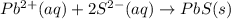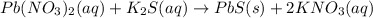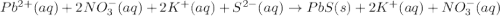Write the net ionic equation (including phases) that corresponds to
pb (no3)2(aq)+k2s(aq)→pbs(...

Chemistry, 28.08.2019 21:00 sarahaziz9526
Write the net ionic equation (including phases) that corresponds to
pb (no3)2(aq)+k2s(aq)→pbs(s)+2kno3(aq)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:50, jordan5778
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
Questions in other subjects:

Physics, 22.04.2021 17:10

Mathematics, 22.04.2021 17:10

Physics, 22.04.2021 17:10


Mathematics, 22.04.2021 17:10



Computers and Technology, 22.04.2021 17:10