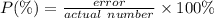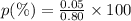Chemistry, 03.11.2020 18:20 annyarias1036
If the observed value for a density is 0.85 g/ml and the accepted value is 0.80 g/ml, what is the percent error? *
0.0625%
0.14%
6.25%
14.%

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

You know the right answer?
If the observed value for a density is 0.85 g/ml and the accepted value is 0.80 g/ml, what is the pe...
Questions in other subjects:

English, 10.02.2020 01:05


Mathematics, 10.02.2020 01:05

Mathematics, 10.02.2020 01:05

Mathematics, 10.02.2020 01:05

History, 10.02.2020 01:05

Mathematics, 10.02.2020 01:05