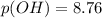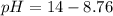A chemist titrates of a aniline solution with HCl solution at . Calculate the pH at equivalence. The of aniline is . Round your answer to decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of solution added.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1

You know the right answer?
A chemist titrates of a aniline solution with HCl solution at . Calculate the pH at equivalence. The...
Questions in other subjects:


Mathematics, 20.10.2020 18:01




Mathematics, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01


History, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01

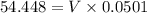
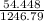 = 0.044 M
= 0.044 M![p(OH)=\frac{1}{2} [pKw+pKb+logC]](/tpl/images/0863/4823/b2809.png)
![p(OH)=\frac{1}{2} [14+4.87+log0.044]](/tpl/images/0863/4823/70b78.png)