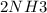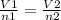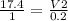Chemistry, 03.11.2020 17:00 diamoniquepowel
A sample of gas contains 0.1000 mol of N2(g) and 0.3000 mol of H2(g) and occupies a volume of 17.4 L. The following reaction takes place: N2(g) + 3H2(g)2NH3(g) Calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

You know the right answer?
A sample of gas contains 0.1000 mol of N2(g) and 0.3000 mol of H2(g) and occupies a volume of 17.4 L...
Questions in other subjects:




History, 20.08.2019 00:30


Mathematics, 20.08.2019 00:30


Mathematics, 20.08.2019 00:30


Mathematics, 20.08.2019 00:30

 →
→