Chemistry, 03.11.2020 16:50 phillipselijah2
Look up the active ingredient in baking soda. Write a molecular and net ionic equation when that active ingredient is mixed with acetic acid. Take note of what blanks have charges to know where to input each chemical. (In the equation input subscripted values as normal text. i. e. water would be H2O)

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:20, JKINGblackstar3502
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1


Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
Look up the active ingredient in baking soda. Write a molecular and net ionic equation when that act...
Questions in other subjects:


History, 25.03.2021 09:40

Mathematics, 25.03.2021 09:40



Mathematics, 25.03.2021 09:40

Chemistry, 25.03.2021 09:50



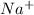 and
and 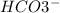
 →
→ 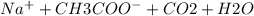
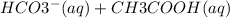 →
→