
Chemistry, 03.11.2020 16:30 petriajack5543
The gas phase reaction between hydrogen and iodine is proposed to occur as follows:step 1fast:I2 2 Istep 2slow:H2 + 2 I 2 HI(1) What is the equation for the overall reaction? Use the smallest integer coefficients possible. If a box is not needed, leave it blank.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, kristieroth1
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 13:10, dookiefadep5n1tt
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
The gas phase reaction between hydrogen and iodine is proposed to occur as follows:step 1fast:I2 2 I...
Questions in other subjects:



English, 29.10.2020 19:50




History, 29.10.2020 19:50



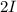 will cancel out and the overall reaction will be
will cancel out and the overall reaction will be 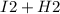 →
→ 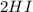
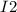 ⇄
⇄  ⇄
⇄ 

