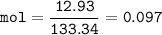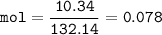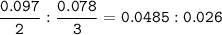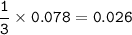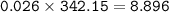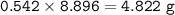Chemistry, 03.11.2020 14:30 krystalhurst97
What mass of Al2(SO4)3 results from mixing 12.93 g of AlCl3 with 10.34 g of (NH4)2SO3 as shown with a 54.2% yield?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 23:00, aly02
Need asap question 1 minerals are organic compounds. true false question 2 what vitamin can be found in foods like oranges, grapefruits, and broccoli? a. vitamin a b. vitamin k c. vitamin c d. vitamin d question 3 what are minerals? a. chemical elements that are needed for body processes. b. organic compounds that the body needs in small amounts to function properly. c. small molecules used to build proteins. d. an organic compound that is insoluble in water and includes fats. question 4 how many types of vitamins does the human body need? a. 15 b. 11 c. 13 d. 17 question 5 vitamins are a good source of energy. true false
Answers: 1


Chemistry, 23.06.2019 04:00, Bassoonist
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
What mass of Al2(SO4)3 results from mixing 12.93 g of AlCl3 with 10.34 g of (NH4)2SO3 as shown with...
Questions in other subjects:

Mathematics, 14.07.2019 04:50

Biology, 14.07.2019 04:50