
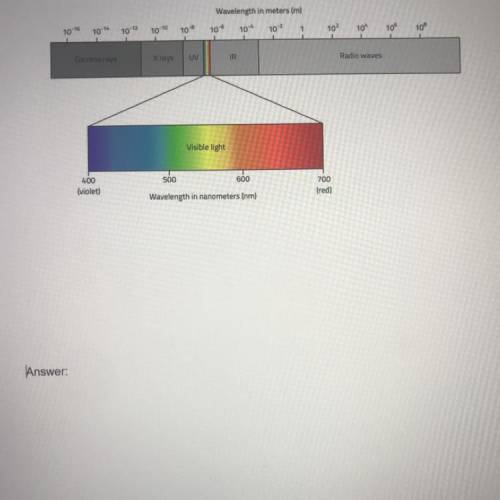
A pair of substances undergoes a change. This change is observed in the form of a difference in energy. Substance A falls from energy level M to energy level N and gives off infrared radiation. Substance B also undergoes a change and has energy that is given off and falls from energy level O to energy level P and gives off ultraviolet radiation. Which transition, from M to N or from O to P, has a greater energy difference? Explain your answer. Use a diagram of the electromagnetic spectrum if necessary.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 14:00, daniel1480
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
A pair of substances undergoes a change. This change is observed in the form of a difference in ener...
Questions in other subjects:


Mathematics, 01.07.2019 22:00


History, 01.07.2019 22:00




Business, 01.07.2019 22:00




