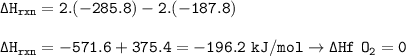Chemistry, 02.11.2020 14:00 steve12335
Comment Both propane and benzene are hydrocarbons. As a rule,
the energy obtained from the combustion of a gram of hydrocar-
bon is between 40 and 50 kJ.
Practice Exercise 1
Calculate the enthalpy change for the reaction
2 H2O2(l)-→ 2 H2O(l) + O2(g)
using enthalpies of formation:
Change in enthalpy(f)H2O2(l)= -187.8 kJ/mol
Change in enthalpy (f) [H2O(l)= -285.8 kJ/mol

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1



Chemistry, 23.06.2019 13:30, medellincolombia99
Which of these statements describes the size of an atom? a. an atom is larger than a sheet of aluminum foil. b. an atom is small but can be seen with just our eyes. c. an atom is the size of a plastic building block. d. an atom is tiny and cannot be seen without magnification.
Answers: 2
You know the right answer?
Comment Both propane and benzene are hydrocarbons. As a rule,
the energy obtained from the combusti...
Questions in other subjects:



Mathematics, 18.06.2020 23:57



Mathematics, 18.06.2020 23:57