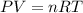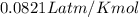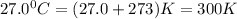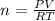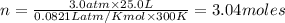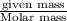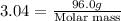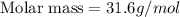Chemistry, 02.11.2020 08:10 GreenHerbz206
The gaseous product of a reaction is collected in a 25.0L container at 27.0 C. The pressure in the container is 3.0atm and the gas has a mass of 96.0g. What is the molar mass of the gas?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, damienlopezram
You 4. you have been swimming in your neighbor’s pool for an hour. the relative humidity of the air is 30 percent. will you feel warm or cool when you step out of the pool? explain your answer.
Answers: 1

Chemistry, 21.06.2019 15:30, BigDough9090
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
The gaseous product of a reaction is collected in a 25.0L container at 27.0 C. The pressure in the c...
Questions in other subjects:

Health, 28.06.2019 19:30


Computers and Technology, 28.06.2019 19:30

Mathematics, 28.06.2019 19:30

Social Studies, 28.06.2019 19:30

Mathematics, 28.06.2019 19:30



Mathematics, 28.06.2019 19:30