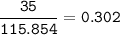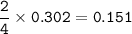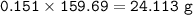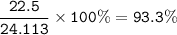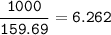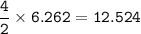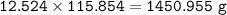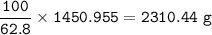Chemistry, 02.11.2020 06:30 Svetakotok
4 FeCO3 + O2 --> 2 Fe2O3 + 4CO2
a) A 35.0 g sample of pure FeCO3 produces 22.5 g of Fe2O3. What is the percentage yield of the reaction?
b) What mass of FeCO3 with a purity of 62.8% is needed to make 1.00 kg of Fe2O3?

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 19:00, elizabethajih99
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
4 FeCO3 + O2 --> 2 Fe2O3 + 4CO2
a) A 35.0 g sample of pure FeCO3 produces 22.5 g of Fe2O3. What...
Questions in other subjects:

Biology, 09.11.2020 18:50

English, 09.11.2020 18:50


Mathematics, 09.11.2020 18:50

Physics, 09.11.2020 18:50

French, 09.11.2020 18:50

Geography, 09.11.2020 18:50

Spanish, 09.11.2020 18:50

History, 09.11.2020 18:50