Someone please help me! I'm so confused on this question! I will give brainliest!
...

Chemistry, 30.10.2020 23:00 lanashanabJHsbd1099
Someone please help me! I'm so confused on this question! I will give brainliest!
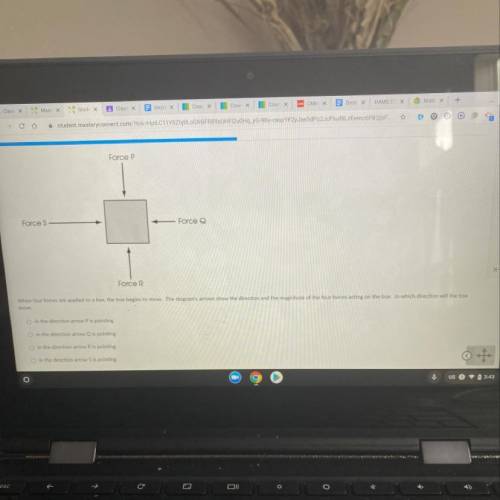

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 23.06.2019 07:20, aprilkenedy12
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1
You know the right answer?
Questions in other subjects:



Biology, 06.10.2019 18:00

Health, 06.10.2019 18:00



Biology, 06.10.2019 18:00

History, 06.10.2019 18:00


Mathematics, 06.10.2019 18:00



