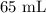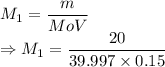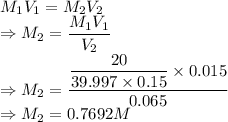Chemistry, 30.10.2020 16:50 kaelah6846
A student prepared a stock solution by dissolving 20.0 g of NaOH in enough water to make 150. mL of solution. She then took 15.0 mL of the stock solution and diluted it with enough water to make 65.0 mL of a final solution. What is the concentration of NaOH for the final solution?
A) O. 411 M
B) 0.534 M
C) 1.87 M
D) 2.43 M

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, reaperqueen21
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

You know the right answer?
A student prepared a stock solution by dissolving 20.0 g of NaOH in enough water to make 150. mL of...
Questions in other subjects:


Chemistry, 17.02.2020 23:46



Mathematics, 17.02.2020 23:46


Social Studies, 17.02.2020 23:47



Mathematics, 17.02.2020 23:47

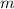 = Mass of sample =
= Mass of sample = 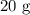
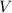 = Volume of solution =
= Volume of solution = 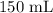
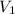 = Initial volume taken out of the stock solution =
= Initial volume taken out of the stock solution = 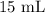
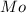 = Molar mass of NaOH =
= Molar mass of NaOH = 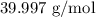
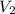 = Final volume of solution =
= Final volume of solution =