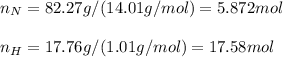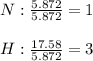a binary compound of nitrogen and hydrogen has the following percentage composition: 82.27% nitrogen; 17.76% hydrogen. If the molar mass of the compound is determined by a separate experiment to be slightly more than 17g what are the empirical and molecular formulas of the compound

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 23:00, emilyphillips1681
If two identical atoms are bonded, what kind of molecule is formed
Answers: 1

Chemistry, 23.06.2019 05:00, MoneyMike42
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
a binary compound of nitrogen and hydrogen has the following percentage composition: 82.27% nitrogen...
Questions in other subjects:

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Social Studies, 11.09.2020 08:01

Chemistry, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01

Mathematics, 11.09.2020 08:01