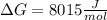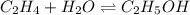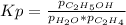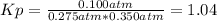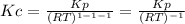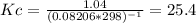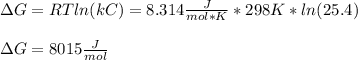Chemistry, 28.10.2020 17:10 Anasiabrown11
g what is ΔG for the reaction at 298K, when the partial pressure of C2H4 is 0.275 atm, the partial pressure of H2O is 0.350 atm, and the partial pressure of C2H5OH is 0.100 atm?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 23.06.2019 08:50, leah5981
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas. caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible. relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3
You know the right answer?
g what is ΔG for the reaction at 298K, when the partial pressure of C2H4 is 0.275 atm, the partial...
Questions in other subjects:


History, 04.02.2020 08:53

Mathematics, 04.02.2020 08:53

Mathematics, 04.02.2020 08:53


History, 04.02.2020 08:53

Mathematics, 04.02.2020 08:53

English, 04.02.2020 08:53