
Chemistry, 28.10.2020 17:00 ummmmmmmmmmmm
An iron ore sample contains plus other impurities. A 896-g sample of impure iron ore is heated with excess carbon, producing 182 g of pure iron by the following reaction: What is the mass percent of FeO2 in the impure iron ore sample

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 10:00, youngchapo813p8d9u1
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 22:30, kiera2599
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 22.06.2019 23:00, chastineondre7979
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
An iron ore sample contains plus other impurities. A 896-g sample of impure iron ore is heated with...
Questions in other subjects:




World Languages, 14.06.2021 21:40

Mathematics, 14.06.2021 21:40

Mathematics, 14.06.2021 21:40



Mathematics, 14.06.2021 21:40

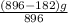 × 100
× 100 

