

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 21:00, cxttiemsp021
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
The pH of a solution of 19.5 g of malonic acid in 0.250 L is 1.47.The pH of a 0.300 M Solution of so...
Questions in other subjects:

Health, 03.09.2020 03:01

Mathematics, 03.09.2020 03:01




Mathematics, 03.09.2020 03:01


Biology, 03.09.2020 03:01

World Languages, 03.09.2020 03:01

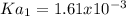
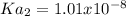
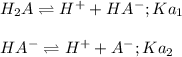
![Ka_1=\frac{[H^+][HA^-]}{[H_2A]}](/tpl/images/0847/3563/40a10.png)
![[H^+]=[HA^-]=10^{-pH}=10^{-1.47}=0.0339M](/tpl/images/0847/3563/389eb.png)
![[H_2A]=\frac{19.5g/(104.06 g/mol)}{0.250L}=0.750M](/tpl/images/0847/3563/b8a4c.png)
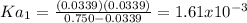
![[H^+]=[A^-^2]=10^{-4.26}=5.50x10^{-5}M](/tpl/images/0847/3563/18ffb.png)
![Ka_2=\frac{[H^+][A^{-2}]}{[HA^-]}=\frac{(5.50x10^{-5})(5.50x10^{-5})}{0.300-(5.50x10^{-5})}\\ \\Ka_2=1.01x10^{-8}](/tpl/images/0847/3563/0e92d.png)


