The coinage metals, Cu, Ag, and Au, all lie
in one column of the periodic table. Consider
Ag....

Chemistry, 28.10.2020 14:00 trevorhenyan51
The coinage metals, Cu, Ag, and Au, all lie
in one column of the periodic table. Consider
Ag. An atom of Ag will have a certain ground
state electron configuration according to the
Aufbau principle. The outermost electrons in
Ag will have the configuration
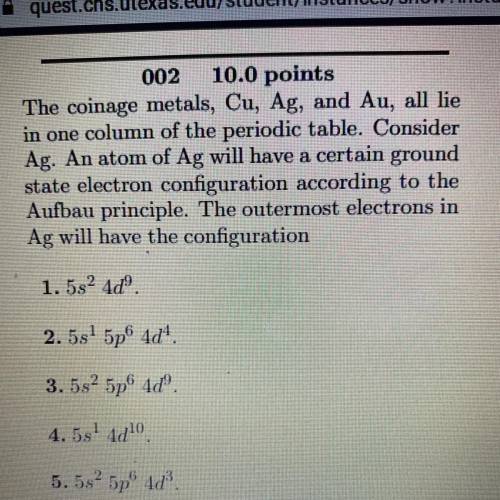

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 03:30, jennelledenise
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1

Chemistry, 23.06.2019 06:30, nikeahbrown
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3

Chemistry, 23.06.2019 09:00, littlemoneyh
20 grams of water. she poured out 15 grams. which of the following physical properties of the water changes? a .boiling point b. density c .electrical conductivity d .volume
Answers: 2
You know the right answer?
Questions in other subjects:

Computers and Technology, 28.06.2019 07:30


History, 28.06.2019 07:30

Computers and Technology, 28.06.2019 07:30

Mathematics, 28.06.2019 07:30

English, 28.06.2019 07:30

History, 28.06.2019 07:30


English, 28.06.2019 07:30



