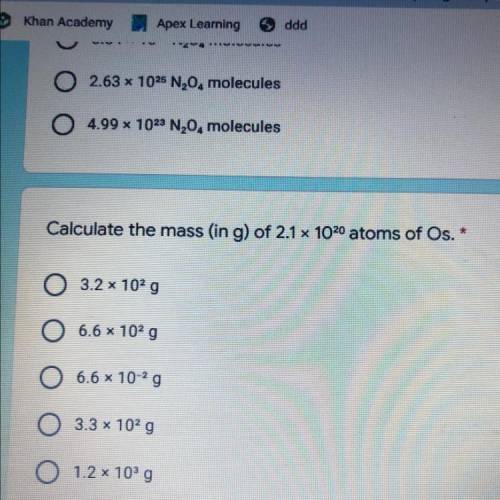
Calculate the mass (in g) of 2.1 x 1020 atoms of Os.
...

Chemistry, 28.10.2020 09:00 trent1002brown
Calculate the mass (in g) of 2.1 x 1020 atoms of Os.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, ulilliareinhart2
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

You know the right answer?
Questions in other subjects:

Biology, 29.08.2019 11:30

Business, 29.08.2019 11:30




Health, 29.08.2019 11:30


Chemistry, 29.08.2019 11:30




