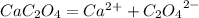Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, tiwaribianca475
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2


Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
Calcium oxalate (cac2o4) has a ksp value of 2.3 × 10–9 at 25°c. calculate the molar solubility of ca...
Questions in other subjects:

Mathematics, 24.02.2021 23:40

Chemistry, 24.02.2021 23:40

Mathematics, 24.02.2021 23:40

Law, 24.02.2021 23:40





Mathematics, 24.02.2021 23:40