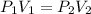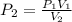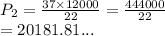Chemistry, 27.10.2020 18:00 cassiuspricerules
A gas sample in a Boyle’s law apparatus is compressed to a volume of 22.0 mL. If its original volume was 37.0 mL at 12,000 Pa, what is the new pressure of the gas sample. Show your calculations.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, ashleymer384
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3

Chemistry, 23.06.2019 00:30, rose888829
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2


Chemistry, 23.06.2019 09:30, kleathers97
Hey, could someone me answer this? much appreciated!
Answers: 1
You know the right answer?
A gas sample in a Boyle’s law apparatus is compressed to a volume of 22.0 mL. If its original volume...
Questions in other subjects:

Mathematics, 02.04.2021 23:30


Mathematics, 02.04.2021 23:30

Mathematics, 02.04.2021 23:30

Mathematics, 02.04.2021 23:30


Mathematics, 02.04.2021 23:40

Mathematics, 02.04.2021 23:40

Mathematics, 02.04.2021 23:40

Mathematics, 02.04.2021 23:40