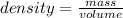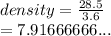Chemistry, 27.10.2020 17:20 Ghhkgu5120
28.5 g of iron shot is added to a graduated cylinder contains 45.50 ml pf water. The water level rises to the 49.10 ml mark from this information calculate the denisty of iron

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 01:30, giraffegurl
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 03:30, ilizzy1224
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
28.5 g of iron shot is added to a graduated cylinder contains 45.50 ml pf water. The water level ris...
Questions in other subjects:

Mathematics, 25.10.2020 04:00

Mathematics, 25.10.2020 04:00

Business, 25.10.2020 04:00

English, 25.10.2020 04:00

English, 25.10.2020 04:00



Mathematics, 25.10.2020 04:00


Mathematics, 25.10.2020 04:00