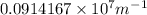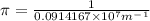Chemistry, 26.10.2020 22:50 oliviaprejean18
Calculate the wavelength of light (in nm) of the spectral line of Hydrogen where an electron falls from the 6th Bohr orbit to the 3rd Bohr orbit.
a) 540 nm
b) 2000 nm
c) 1090 nm
d) 1050 nm

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, logan12345677885675
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2


Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 12:30, MrSavannahCat
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1
You know the right answer?
Calculate the wavelength of light (in nm) of the spectral line of Hydrogen where an electron falls f...
Questions in other subjects:

Mathematics, 12.01.2021 17:40




Mathematics, 12.01.2021 17:40


Mathematics, 12.01.2021 17:40

English, 12.01.2021 17:40


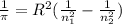
![[n_2n_1]](/tpl/images/0841/8494/b45f5.png)
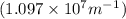
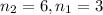
 wavelength of light
wavelength of light 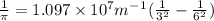
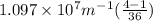
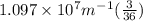
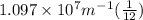
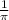 =
=