Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
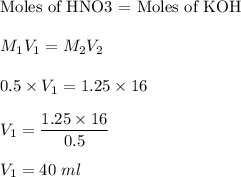
In a titration, what volume of 0.50 M HNO3 is required to completely react with 16.0 mL of 1.250 M K...
Questions in other subjects:


Mathematics, 23.08.2020 21:01


Mathematics, 23.08.2020 21:01

Arts, 23.08.2020 21:01

Social Studies, 23.08.2020 21:01

Mathematics, 23.08.2020 21:01

History, 23.08.2020 21:01