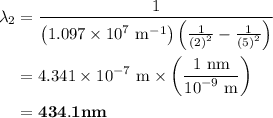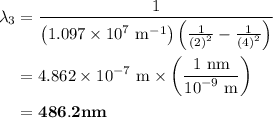Chemistry, 17.09.2019 12:20 lizzyhearts
The spectral lines observed for hydrogen arise from transitions from excited states back to the n=2 principle quantum level. calculate the wavelengths associated with the spectral transitions of the hydrogen atom from the n=6,5,4 and 3 to the n=2 level.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, colochaortiz20p7cajw
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 07:30, reaperqueen21
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 19:30, jessixa897192
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
The spectral lines observed for hydrogen arise from transitions from excited states back to the n=2...
Questions in other subjects:



Mathematics, 23.07.2019 18:00

History, 23.07.2019 18:00


Mathematics, 23.07.2019 18:00





 = Rydberg constant =
= Rydberg constant = 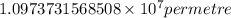
 = wavelength
= wavelength and
and  are the level of transitions.
are the level of transitions.


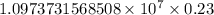
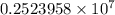



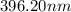



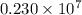



















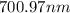
 .
.
 .
.
 .
.
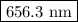 .
.
 …… (1)
…… (1) is the Rydberg constant that has the value
is the Rydberg constant that has the value 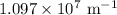 ,
,  is the initial energy level of transition, and
is the initial energy level of transition, and  is the final energy level of transition.
is the final energy level of transition.
 …… (2)
…… (2)