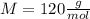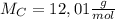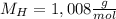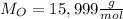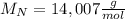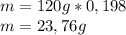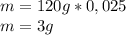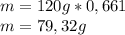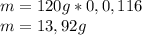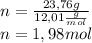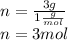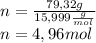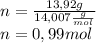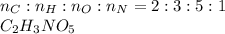Chemistry, 22.10.2020 14:01 pizarroisaid
Peroxyacylnitrate (PAN) is one of the components
of smog. It is a compound of C, H, N, and O.
Determine the percent composition of oxygen and
the empirical formula from the following percent
composition by mass: 19.8 percent C,
2.50 percent
H, 11.6 percent N. What is its molecular formula
given that its molar mass is about 120 g?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, sgslayerkingminecraf
Which of the following statements about acidic water is true? a. acid has no effect on the h, o molecules. b. the solution contains a larger number of oh ions than h, o ions. c. the solution contains a larger number of h, o ions than qh ions. d. the solution contains an equal number of h, o ions and oh ions. none of the above e.
Answers: 1


Chemistry, 22.06.2019 19:30, toriabrocks
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
You know the right answer?
Peroxyacylnitrate (PAN) is one of the components
of smog. It is a compound of C, H, N, and O.
...
...
Questions in other subjects:

Mathematics, 18.02.2021 22:50

Social Studies, 18.02.2021 22:50




Mathematics, 18.02.2021 22:50



Mathematics, 18.02.2021 22:50