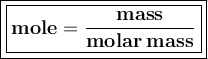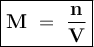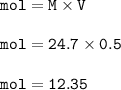Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 23.06.2019 09:30, kleathers97
Hey, could someone me answer this? much appreciated!
Answers: 1


Chemistry, 23.06.2019 19:30, lillygon81
Given ch4+2o2—> co2+2h2o what mass of oxygen would be needed to form 17 grams of carbon dioxide
Answers: 1
You know the right answer?
One of the uses of methanol (CH3OH) in dilute form is as a windshield washer antifreeze. In pure for...
Questions in other subjects:



Computers and Technology, 26.03.2021 01:30


Mathematics, 26.03.2021 01:30

Health, 26.03.2021 01:30


Mathematics, 26.03.2021 01:30