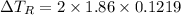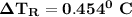Chemistry, 21.10.2020 16:01 hannahpelkey
Calculate the freezing point of a solution containing 10 grams of KCl and 1100.0 grams of water. The molal freezing point depression constant (Kf) for water is 1.86

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, alejandra1201
10. according to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
Answers: 3


Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2
You know the right answer?
Calculate the freezing point of a solution containing 10 grams of KCl and 1100.0 grams of water. The...
Questions in other subjects:

Mathematics, 18.02.2020 04:37




Mathematics, 18.02.2020 04:38



Spanish, 18.02.2020 04:38

Health, 18.02.2020 04:38

Mathematics, 18.02.2020 04:38

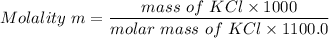
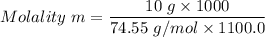
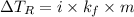
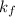 = 1.86° C/m
= 1.86° C/m