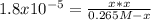Chemistry, 21.10.2020 05:01 maricruzisfye
Consider the reaction: CH3COOH(aq)+H2O(l)⇌H3O+(aq)+CH3COO− (aq) K=1.8×10−5 at 25∘C Part A If a solution initially contains 0.265 molL−1 CH3COOH, what is the equilibrium concentration of H3O+ at 25∘C?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
Consider the reaction: CH3COOH(aq)+H2O(l)⇌H3O+(aq)+CH3COO− (aq) K=1.8×10−5 at 25∘C Part A If a solut...
Questions in other subjects:


History, 15.02.2021 07:10

Biology, 15.02.2021 07:10

Mathematics, 15.02.2021 07:10



History, 15.02.2021 07:10

Mathematics, 15.02.2021 07:10


![[H_3O]^+=2.18x10^{-3}M](/tpl/images/0827/0667/36cd6.png)
![Ka=\frac{[H_3O^+][CH_3COO^-]}{[CH_3COOH]}](/tpl/images/0827/0667/570cb.png)
 based on the ICE table, in which it equals the concentration of both H3O+ and CH3COO-, we can also write:
based on the ICE table, in which it equals the concentration of both H3O+ and CH3COO-, we can also write: