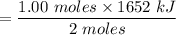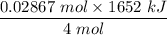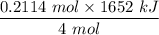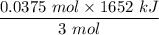Chemistry, 20.10.2020 20:01 channarlawassociate
The overall reaction in a commercial heat pack can be represented as How much heat is released when 4.40 moles of iron are reacted with excess ? Heat = kJ How much heat is released when 1.00 mole of is produced? Heat = kJ How much heat is released when 1.60 g iron is reacted with excess ? Heat = kJ How much heat is released when 11.8 g and 1.20 g are reacted? Heat = kJ

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 23:00, emilyphillips1681
If two identical atoms are bonded, what kind of molecule is formed
Answers: 1
You know the right answer?
The overall reaction in a commercial heat pack can be represented as How much heat is released when...
Questions in other subjects:


Mathematics, 28.08.2020 21:01

Advanced Placement (AP), 28.08.2020 21:01

Mathematics, 28.08.2020 21:01

Social Studies, 28.08.2020 21:01



Chemistry, 28.08.2020 21:01


Mathematics, 28.08.2020 21:01

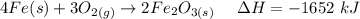
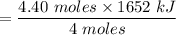
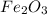 is produced?
is produced?