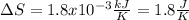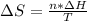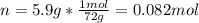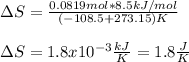Chemistry, 20.10.2020 18:01 ausemkattom3034
he heat of fusion of tetrahydrofuran is . Calculate the change in entropy when of tetrahydrofuran melts at . Be sure your answer contains a unit symbol. Round your answer to significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, tiwaribianca475
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2


Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1
You know the right answer?
he heat of fusion of tetrahydrofuran is . Calculate the change in entropy when of tetrahydrofuran me...
Questions in other subjects:

Mathematics, 13.09.2020 16:01

Social Studies, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Spanish, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01