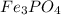Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1


Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1
You know the right answer?
A 13.58 g sample of a compound contains 8.67 g of iron, Fe, 1.60 g of phosphorus, P, and oxygen, O....
Questions in other subjects:



Mathematics, 12.03.2021 21:30



Mathematics, 12.03.2021 21:30




Mathematics, 12.03.2021 21:30