
Chemistry, 18.10.2020 14:01 tinyturtles
Aluminum metal reacts with chlorine gas to form aluminum chloride. The reaction can be represented by the following balanced chemical equation:
2 Al(s) + 3 Cl2(g) → 2 AlCl3(s).
If you were trying to react 52.9 grams of chlorine gas completely, how many grams of aluminum would you need?
[grams of Aluminum] Do NOT enter the unit in your answer. Report your answer with 3 SFs.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
Aluminum metal reacts with chlorine gas to form aluminum chloride. The reaction can be represented b...
Questions in other subjects:










Mathematics, 03.03.2020 03:50

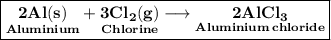
 of Aluminium.
of Aluminium. =
= of Aluminium.
of Aluminium.

