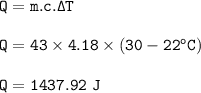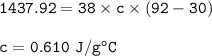Chemistry, 18.10.2020 14:01 skeletonorchestra
When 38 g of a metal at 92 °C is added to
43 g of water at 22°C, the temperature of the
water rises to 30°C. What is the specific heat
capacity of the metal? Assume no heat was
lost to the surroundings.
Answer in units of
J/g degrees C

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2


Chemistry, 22.06.2019 19:40, jholland03
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2
You know the right answer?
When 38 g of a metal at 92 °C is added to
43 g of water at 22°C, the temperature of the
...
43 g of water at 22°C, the temperature of the
...
Questions in other subjects:

Mathematics, 12.04.2021 22:40







Mathematics, 12.04.2021 22:40