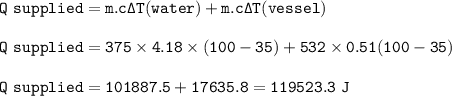Calculate the heat that must be supplied to
a 532 , stainless steel vessel containing 375 g
o...

Chemistry, 18.10.2020 14:01 iziyahh902
Calculate the heat that must be supplied to
a 532 , stainless steel vessel containing 375 g
of water to raise its temperature from 35 C
to the boiling point of water 100°C. Cs. =0.51 J/g. degrees C
Answer in units of J.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 05:30, jalynholden07
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3

Chemistry, 23.06.2019 14:30, Knownothing
2.38g of black copper (ii) oxide is completely reduced by hydrogen to give copper and water. what are the masses of copper and water formed? ?
Answers: 1

Chemistry, 23.06.2019 16:50, alosnomolina1122
Consider the balanced equation below. pcl3+cl2-> pcl5 what is the mole ratio of pcl3 to pcl5
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 12.03.2021 18:30



Mathematics, 12.03.2021 18:30

Arts, 12.03.2021 18:30


English, 12.03.2021 18:30


Mathematics, 12.03.2021 18:30