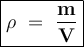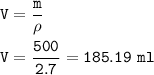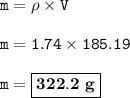Chemistry, 18.10.2020 14:01 hannahboohoo120
A cube of aluminum has a mass of 500g. What will be the mass of a cube of magnesium of the same dimensions? (Density of aluminum is 2.7g/ml)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, kangasc6124
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1


Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
A cube of aluminum has a mass of 500g. What will be the mass of a cube of magnesium of the same dime...
Questions in other subjects:


Mathematics, 20.01.2021 21:10

Advanced Placement (AP), 20.01.2021 21:10

Mathematics, 20.01.2021 21:10

History, 20.01.2021 21:10

Biology, 20.01.2021 21:10

Mathematics, 20.01.2021 21:10


Mathematics, 20.01.2021 21:10