
Chemistry, 18.10.2020 15:01 imstressed
Sodium reacts violently in chlorine gas to form sodium chloride. If 2.5 mol Na and 1.1 mol Cl2 are placed in a flask and allowed to react, how much sodium chloride could be formed?
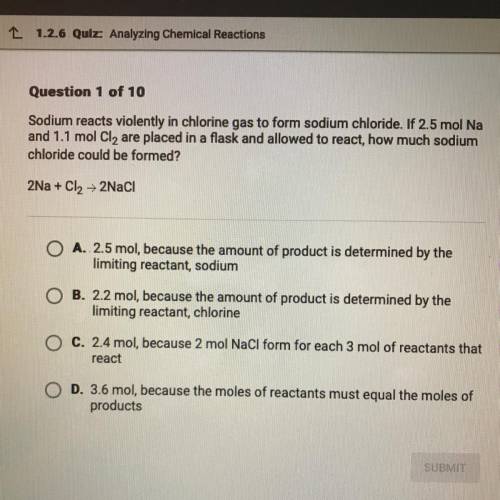

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2


Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
Sodium reacts violently in chlorine gas to form sodium chloride. If 2.5 mol Na and 1.1 mol Cl2 are p...
Questions in other subjects:

Biology, 04.10.2019 18:30





Biology, 04.10.2019 18:30


Mathematics, 04.10.2019 18:30

Mathematics, 04.10.2019 18:30

Mathematics, 04.10.2019 18:30



