
Chemistry, 18.10.2020 15:01 lucaszsanches
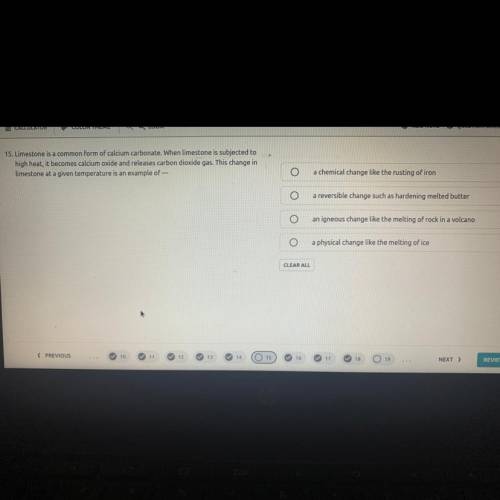
Limestone is a common form of calcium carbonate. When limestone is subjected to
high heat, it becomes calcium oxide and releases carbon dioxide gas. This change in
Limestone at a given temperature is an example of ?

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

You know the right answer?
Limestone is a common form of calcium carbonate. When limestone is subjected to
high heat, it becom...
Questions in other subjects:


Biology, 24.06.2019 22:50

History, 24.06.2019 22:50

Mathematics, 24.06.2019 22:50

Mathematics, 24.06.2019 22:50


English, 24.06.2019 22:50

Biology, 24.06.2019 22:50




