Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3


Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 20:30, demarcuswiseman
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
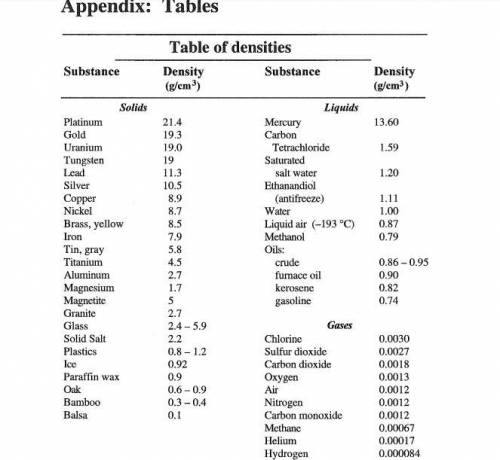
A student measures the mass and volume of a sample of aluminum at room temperature, and calculates t...
Questions in other subjects:




Mathematics, 15.04.2021 20:10

Physics, 15.04.2021 20:10



History, 15.04.2021 20:10