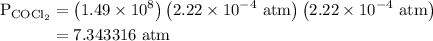Chemistry, 29.09.2019 09:00 charae0185
The kp for the reaction below is 1.49 × 108 at 100.0°c: co(g) + cl2(g) → cocl2(g) in an equilibrium mixture of the three gases, pco = pcl2 = 2.22 × 10-4 atm. the partial pressure of the product, phosgene (cocl2), is atm.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, daniel1480
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced. be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 22:30, wpatskiteh7203
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
The kp for the reaction below is 1.49 × 108 at 100.0°c: co(g) + cl2(g) → cocl2(g) in an equilibrium...
Questions in other subjects:

Mathematics, 17.11.2020 18:30


History, 17.11.2020 18:30


Mathematics, 17.11.2020 18:30

Mathematics, 17.11.2020 18:30


French, 17.11.2020 18:30

Biology, 17.11.2020 18:30

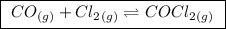 (a balanced reaction)
(a balanced reaction)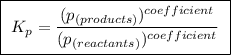
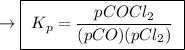
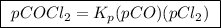
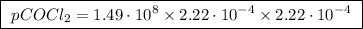
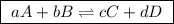 ,
,![\boxed{ \ K_c = \frac{[C]^c[D^d]}{[A]^a[B]^b} \ }](/tpl/images/0273/3667/d057b.png)
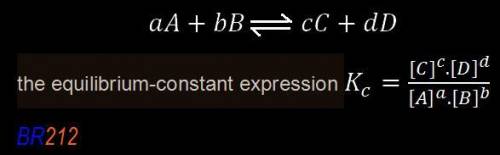
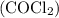 is
is 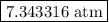 .
.
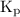 .
.
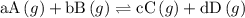
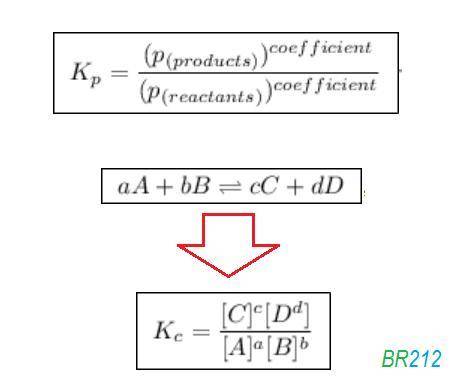
![{{\text{K}}_{\text{p}}} = \dfrac{{{{\left[ {{{\text{P}}_{\text{C}}}} \right]}^{\text{c}}}{{\left[ {{{\text{P}}_{\text{D}}}} \right]}^{\text{d}}}}}{{{{\left[ {{{\text{P}}_{\text{A}}}} \right]}^{\text{a}}}{{\left[ {{{\text{P}}_{\text{B}}}} \right]}^{\text{b}}}}}](/tpl/images/0273/3667/ee3bd.png)
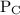 and
and 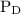 are partial pressures of C and D respectively.
are partial pressures of C and D respectively.
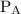 and
and 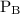 are partial pressures of A and B respectively.
are partial pressures of A and B respectively.
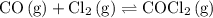
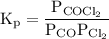 ...... (1)
...... (1)
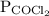 is partial pressure of
is partial pressure of 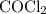 .
.
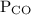 is partial pressure of CO.
is partial pressure of CO.
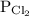 is partial pressure of
is partial pressure of 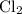 .
.
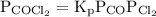 …… (2)
…… (2)
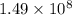 for
for 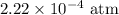 for
for