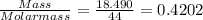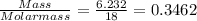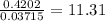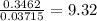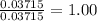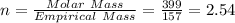Chemistry, 29.08.2019 12:10 cdvazquez727
When a sample of a compound in the vitamin d family was burned in a combustion analysis, 5.983 mg of the compound gave 18.490 mg of co2 and 6.232 mg of h2o. this compound was found to have a molecular mass of 399. what is the molecular formula of this compound? put your answer in form of cxhyoz.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, ReveenatheRaven2296
Which type of reaction always has an element and a compound as reactants
Answers: 1


Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
When a sample of a compound in the vitamin d family was burned in a combustion analysis, 5.983 mg of...
Questions in other subjects:


Mathematics, 26.03.2020 22:06

Mathematics, 26.03.2020 22:06

Mathematics, 26.03.2020 22:06


Mathematics, 26.03.2020 22:06


English, 26.03.2020 22:06

History, 26.03.2020 22:06