
Chemistry, 16.10.2020 17:01 arielpraczko1
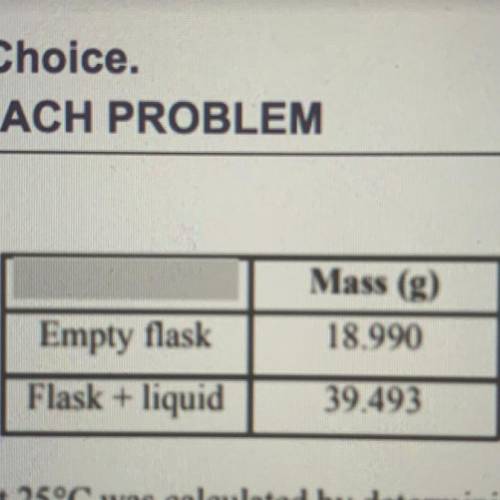
The density of a pure liquid at 25 degrees Celsius was calculated by determining the mass and volume of a sample of the liquid. A student measured the mass of a clean, dry 25.00 mL volumetric flask, filled the flask to its calibration mark with the liquid, and then measured the mass of the flask and liquid. The recorded measurements are shown in the table above. On the basis of this information, to how many significant figures should the density of the liquid be reported?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:40, babygirlqueen5588
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 00:00, aubreymoore9441
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 23.06.2019 01:00, birdman2540
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
The density of a pure liquid at 25 degrees Celsius was calculated by determining the mass and volume...
Questions in other subjects:


Arts, 03.09.2021 20:00


Social Studies, 03.09.2021 20:00

Mathematics, 03.09.2021 20:00

Mathematics, 03.09.2021 20:00


History, 03.09.2021 20:00

Mathematics, 03.09.2021 20:00

Mathematics, 03.09.2021 20:00



