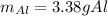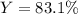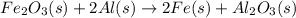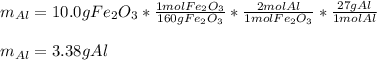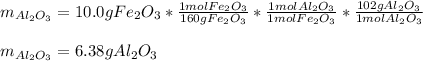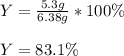Chemistry, 16.10.2020 03:01 jesusmojica25
The thermite reaction occurs when iron(III) oxide reacts with solid
aluminum. The reaction is so hot that molten iron forms as a product.
>
Fe2O3(s) + Al(s) → Fe(C) + Al2O3(s)
What mass of aluminum should be used in order to completely
consume 10.0 g Fe2O3(s)? If the reaction described produces 5.3 g
Al2O3(s), what is the percent yield?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, 20jessicacabriales
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 08:30, waterborn7152
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
The thermite reaction occurs when iron(III) oxide reacts with solid
aluminum. The reaction is so ho...
Questions in other subjects:


Biology, 15.11.2020 17:00

Mathematics, 15.11.2020 17:00

Chemistry, 15.11.2020 17:00


Health, 15.11.2020 17:00



English, 15.11.2020 17:00

English, 15.11.2020 17:00