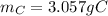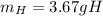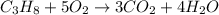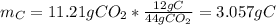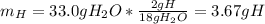Help I need to show work!
Question: The result of a reaction of propane and air gave 11.21 g CO2 and 33.0 grams water according
to the following equation: C3H3 + 5 02 + 3 CO2 + 4 H20
a) What type of reaction (name reaction) is this?
b. How many grams of carbon were produced?
C. How many grams of hydrogen were produced?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 23.06.2019 03:00, rayne40
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

Chemistry, 23.06.2019 03:00, amberskids2
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2

Chemistry, 23.06.2019 09:00, msladycie8831
Avogradoa number was calculated by determining the number of atoms in?
Answers: 1
You know the right answer?
Help I need to show work!
Question: The result of a reaction of propane and air gave 11.21 g CO2 an...
Questions in other subjects:

Mathematics, 21.07.2019 04:10

History, 21.07.2019 04:10





Mathematics, 21.07.2019 04:10

Mathematics, 21.07.2019 04:10