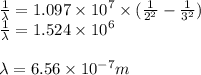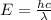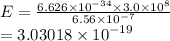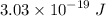Chemistry, 15.10.2020 04:01 itsyaboiamo
For a standard hydrogen atom, how much energy would be released for an electron in the third energy level to fall to the second energy level? Please show all calculations necessary.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, pinkypie123457
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 21.06.2019 20:50, karlyisaunicorn
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2


Chemistry, 22.06.2019 08:30, jalst6084
7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation. 8. when a 2.5 mol of sugar (c12h22o11) are added to a certain amount of water the boiling point is raised by 1 celsius degree. if 2.5 mol of aluminum nitrate is added to the same amount of water, by how much will the boiling point be changed? show all calculations leading to your answer or use 3 – 4 sentences to explain your answer. 9. if 5.40 kcal of heat is added to 1.00 kg of water at 100⁰c, how much steam at 100⁰c is produced? show all calculations leading to an answer. 10. the freezing of water at 0⁰c can be represented as follows: h2o (l) ↔ h2o(s) the density of liquid water is 1.00 g/cm3. the density of ice is 0.92 g/cm3. in 3 – 4 sentences explain why applying pressure causes ice to melt.
Answers: 1
You know the right answer?
For a standard hydrogen atom, how much energy would be released for an electron in the third energy...
Questions in other subjects:










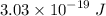
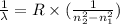
 is the wavelength of the emitted photon
is the wavelength of the emitted photon